Product Details
EEG-CDAs is a clinical web application that embeds deep-learning models into the full EEG workflow. It goes beyond viewing by reshaping the process end to end: multi-device, multi-format acquisition; encrypted transport; automated cleaning and normalization; time-aligned AI analysis; intuitive overlays for rapid review; and hospital-template report drafting for clinician editing and signature. Final results synchronize to HIS/PACS/EMR, and non-DICOM recordings are fully converted to DICOM, yielding a consistent, retrievable, auditable data stream.
Integrated clinical workflow
The journey starts at the recording room, with acquisition on devices, workstations, or tablets. Data travel over HTTPS with TLS/SSL and JWT session control so only authorized sessions can write cases. On arrival, an automated preprocessing pipeline applies linear and non-linear filtering, mitigates motion and EMG artifacts, and normalizes by channel and patient, creating a stable input regime for robust model behavior across real-world variability.
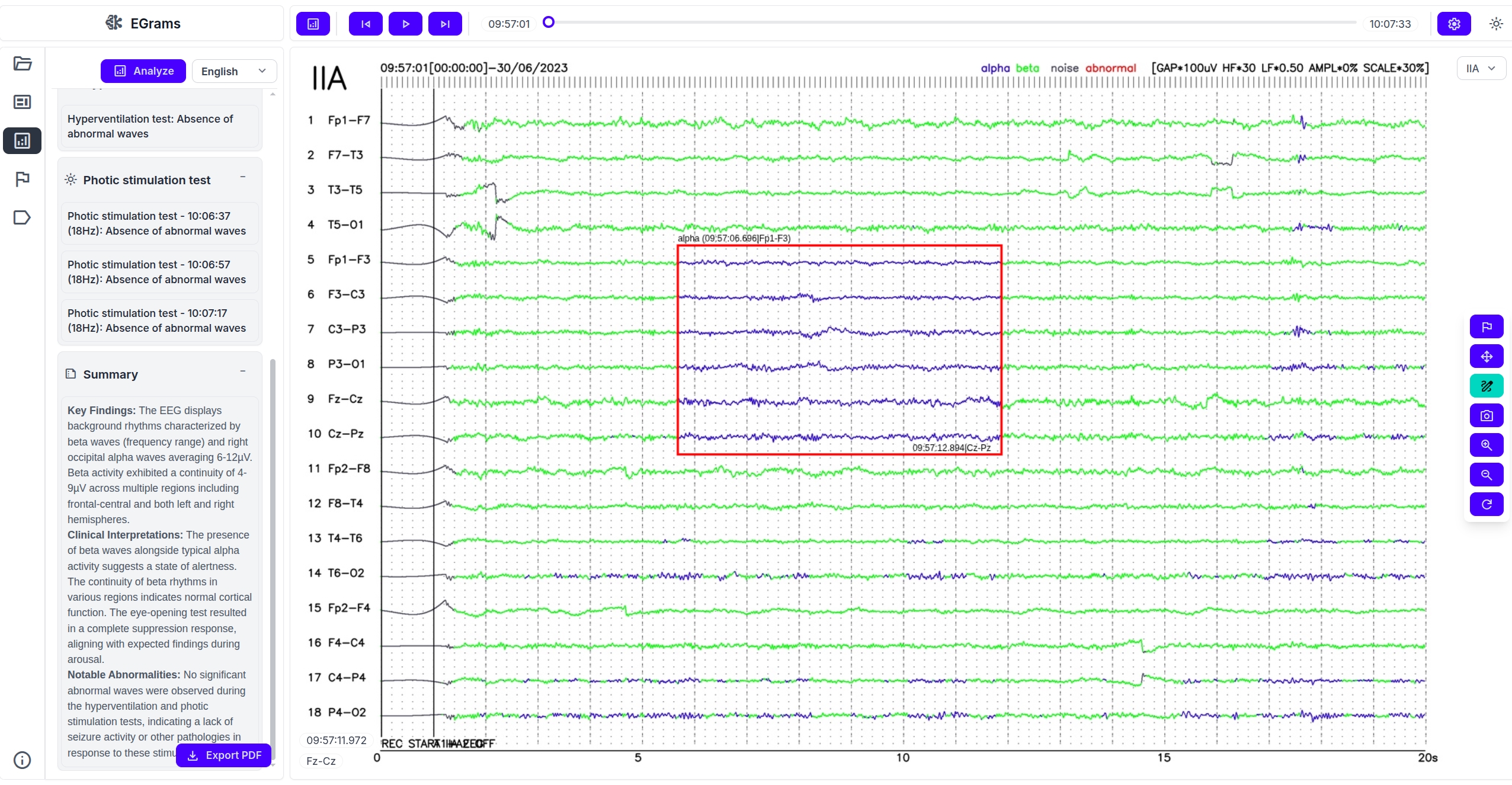
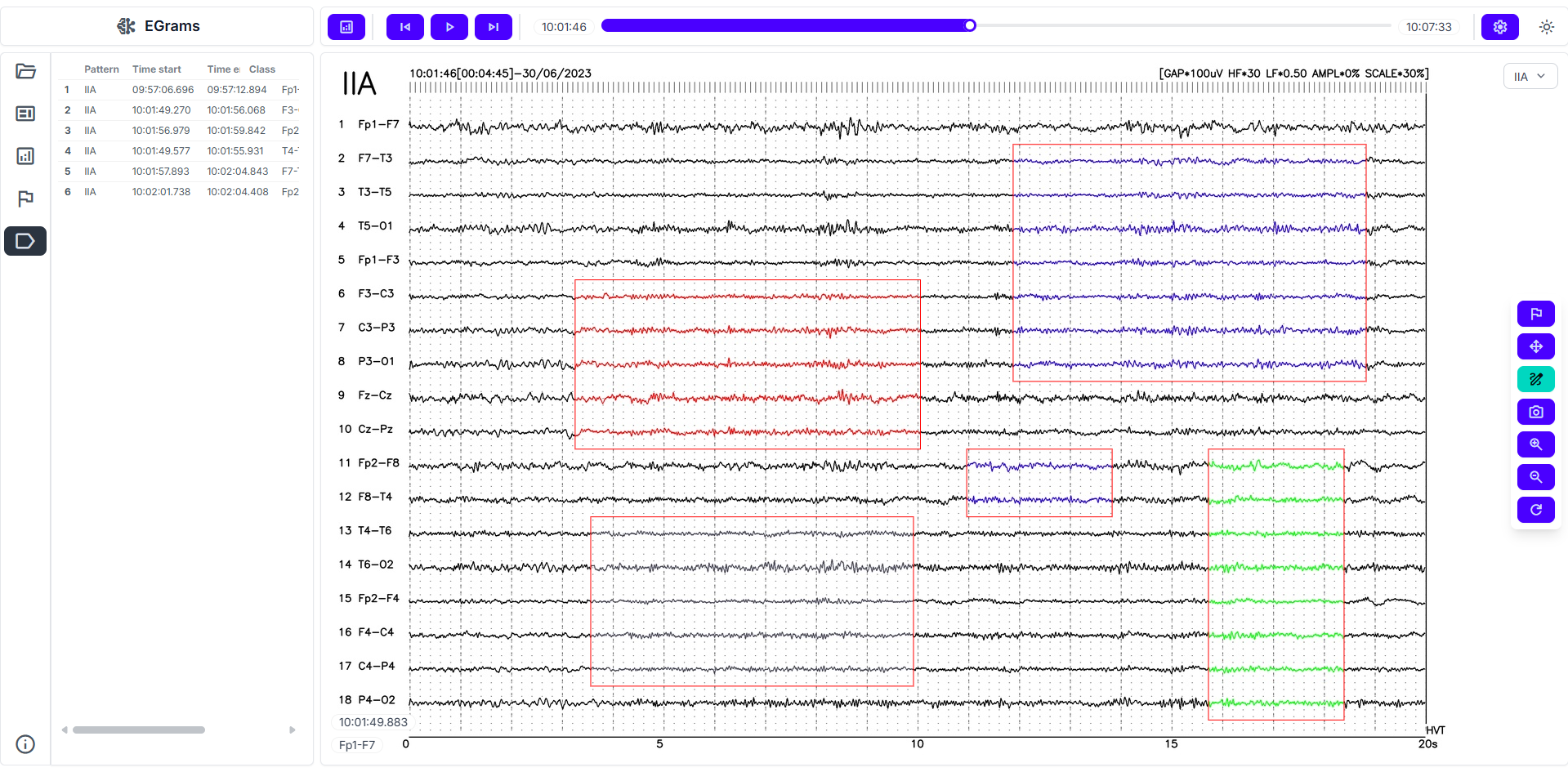
Time-aligned AI analysis
On normalized signals, AI runs 2 complementary tracks. The detection branch scans the timeline for clinically meaningful abnormalities, with emphasis on epileptiform activity. The classification branch assigns alpha/beta/delta/theta bands and tags artifacts or atypical segments. Outputs are routed through a clinical logic layer that prioritizes strong intervals and suppresses weak suggestions, reducing visual fatigue and shortening review time.
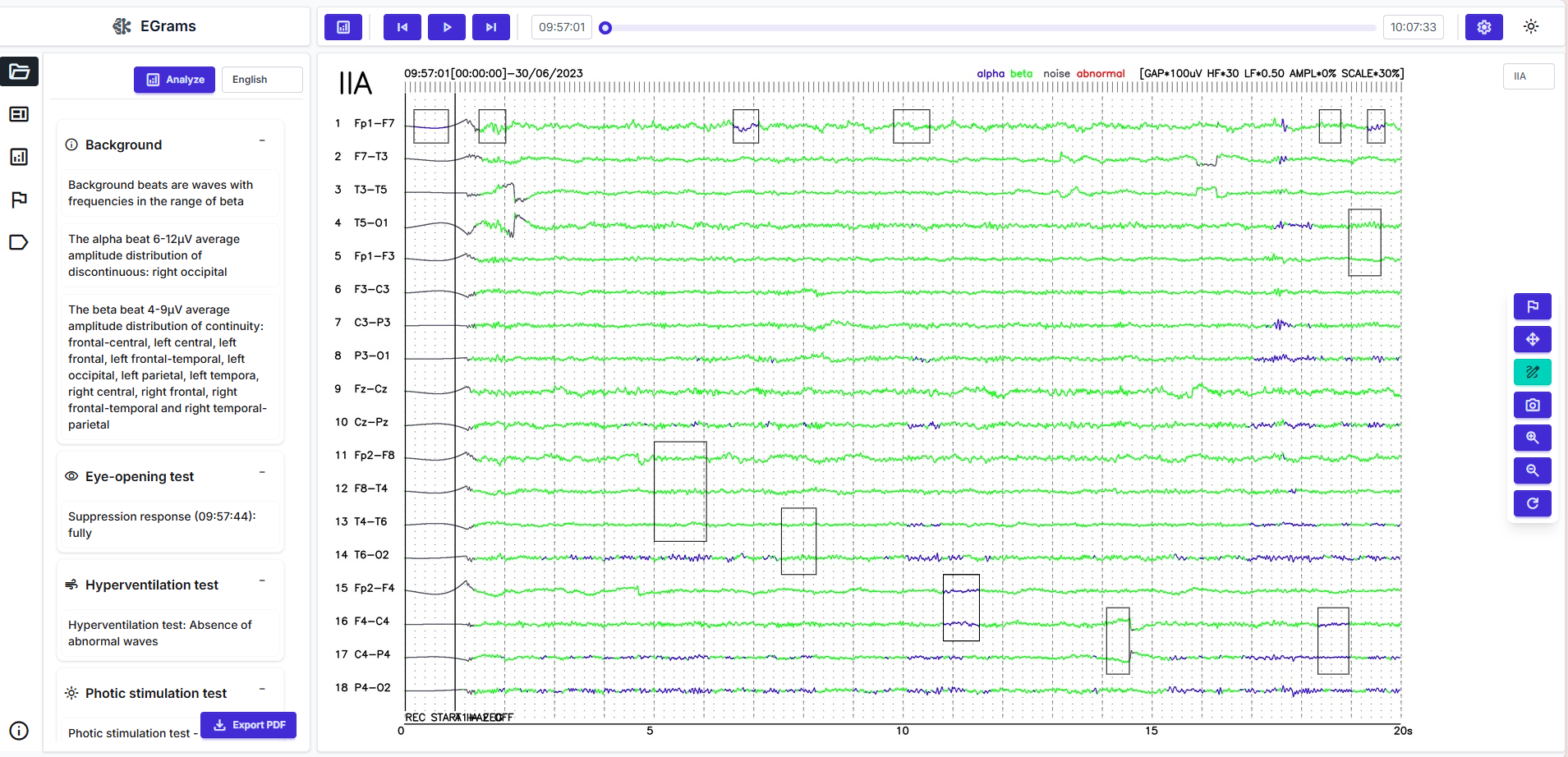
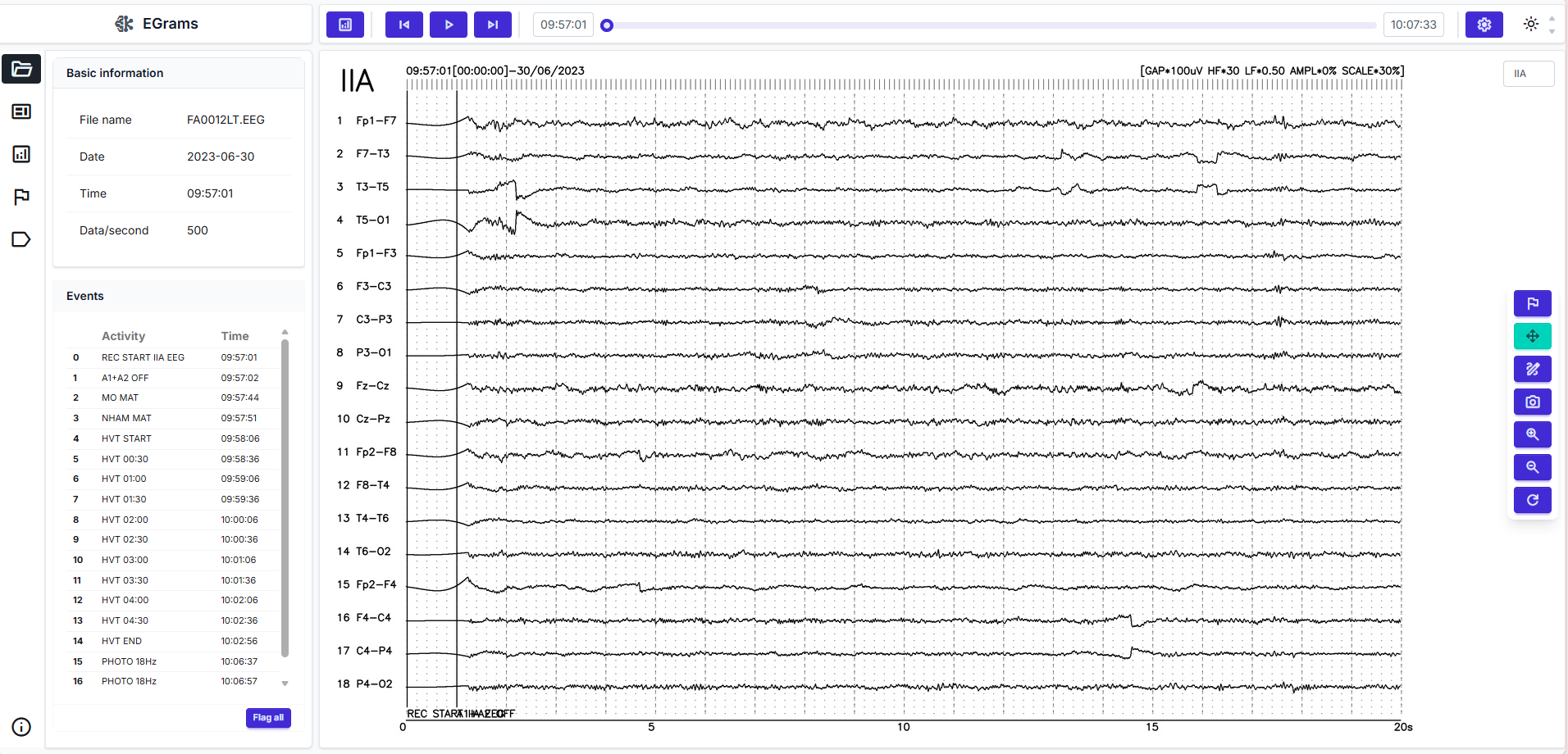
EEG viewer and reading experience
Refined suggestions appear in a multi-channel viewer. Clinicians navigate the timeline, jump via markers or heatmaps, zoom and pan fluidly, and annotate directly on traces. A comparison mode places pre- and post-processing views side by side to ensure filtering has not removed diagnostically relevant content. With adequate hardware, interaction can be near real-time, which is valuable for rapid feedback scenarios.
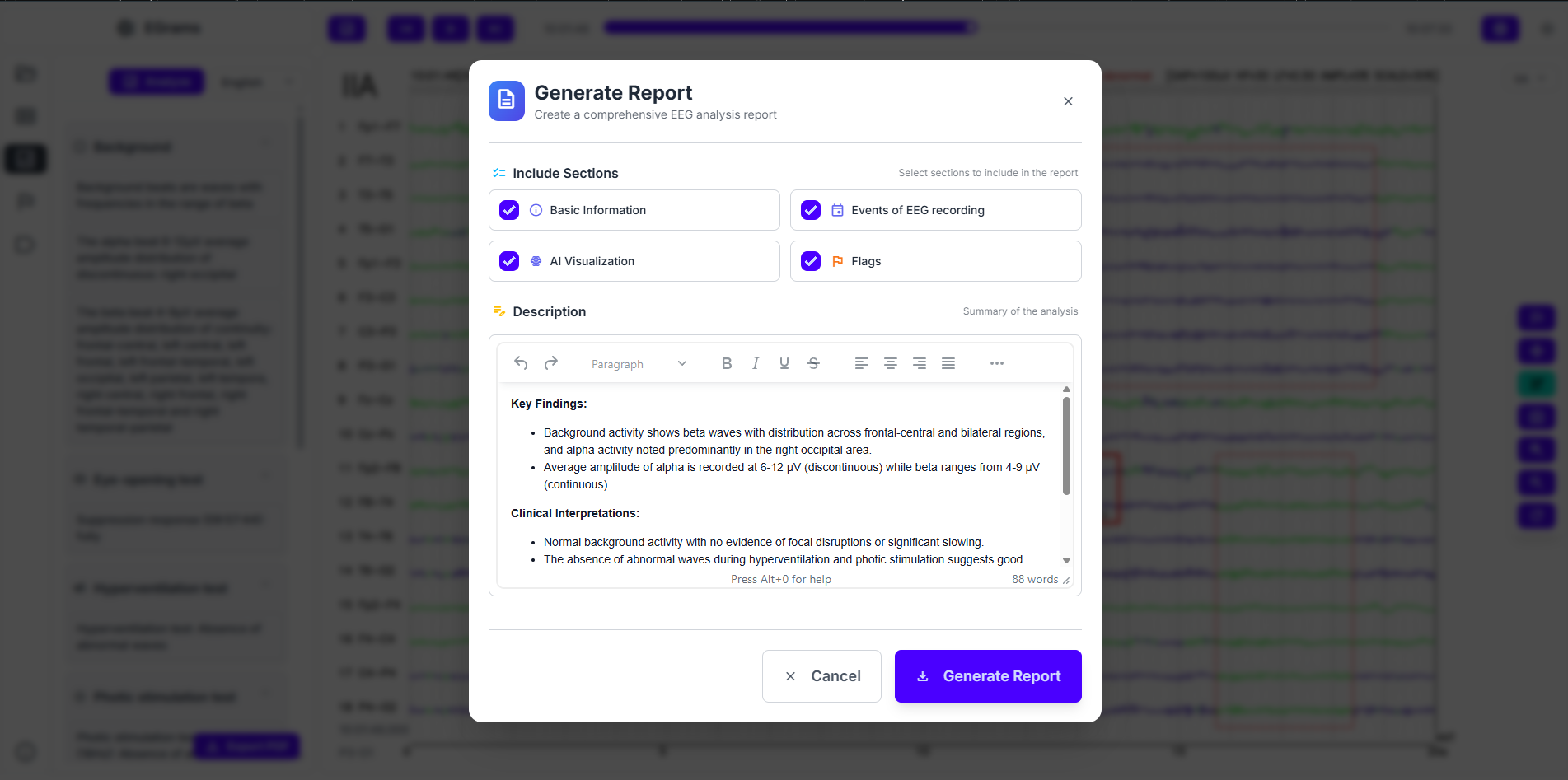
Automated reporting and sign-off
When review is complete, the system drafts a report in the institution’s template with standardized narrative and pre-filled fields from AI results. Control remains with the signer: clinicians edit, conclude, and sign before release. The signed report and related artifacts synchronize to HIS/PACS/EMR with full versioning and access traces for governance and quality programs.
System integration and DICOM interoperability
EEG-CDAs includes a non-DICOM→DICOM conversion pipeline for 100% of pilot cases, placing EEG alongside other modalities within PACS. Secure APIs push reports, metadata, and links to hospital servers and synchronize hospital databases for retrieval, statistics, and cross-department workflows. Standardization enables quality dashboards, KPI monitoring, and internal training.
Architecture, scale, and operations
The platform is built for horizontal scale. The AI cluster can add GPU nodes and increase inference workers as volume grows, while a load balancer maintains responsiveness and availability. A centralized EEG repository stores raw signals, normalized AI outputs, and de-identified metadata, supporting longitudinal follow-up and iterative calibration so accuracy is sustained as device mixes or artifact profiles evolve. All connections enforce authentication, role-based authorization, and audit logging.

Measurable outcomes and clinical value
Pilot KPIs include average per-case turnaround time, accuracy on core tasks (abnormality detection and rhythm classification), DICOM conversion success rate, system uptime, and user satisfaction. Targets are to cut reading time from ~40–45 minutes to ~4 minutes per case, reach 94–98% accuracy depending on task, and achieve 100% conversion and synchronization for pilot cases.
Representative scenarios
For first-time epilepsy assessments, AI overlays guide attention to candidate epileptiform intervals and the drafted narrative shortens the path to a polished report. At follow-ups, prior EEGs are recalled from the centralized repository to compare trajectories, while the drafting module resurfaces salient context to keep longitudinal narratives consistent. In multidisciplinary conferences, DICOM-converted data enable PACS-wide viewing with full access logs for compliance.